Org. Biomol. Chem., 2023, Advance Article
DOI: 10.1039/D3OB01120H, Communication
DOI: 10.1039/D3OB01120H, Communication
Zheyao Li, Chunmei Ma, Lin Zhao, Zhongren Lin, Yang Hu, Jianhong Zhao, Xinhong Yu
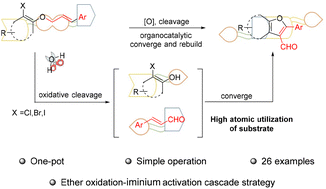
This work reported a method for conversion of aryl allyl ethers into furancarbaldehydes via an ether oxidation iminium-ion activation cascade strategy. The method features simple operation, mild conditions, and especially high atomic utilization.
To cite this article before page numbers are assigned, use the DOI form of citation above.
The content of this RSS Feed (c) The Royal Society of Chemistry
This work reported a method for conversion of aryl allyl ethers into furancarbaldehydes via an ether oxidation iminium-ion activation cascade strategy. The method features simple operation, mild conditions, and especially high atomic utilization.
To cite this article before page numbers are assigned, use the DOI form of citation above.
The content of this RSS Feed (c) The Royal Society of Chemistry


 Open Access
Open Access