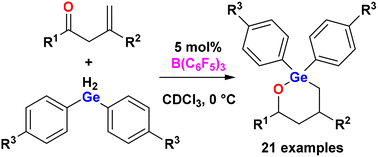
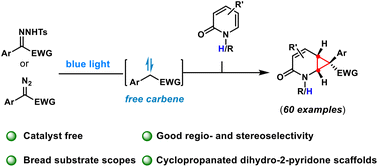
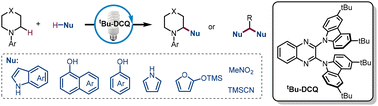
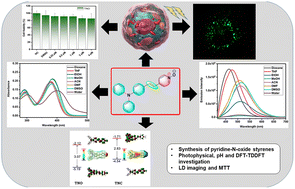
Org. Biomol. Chem., 2023, Advance Article
DOI: 10.1039/D3OB01312J, Communication
DOI: 10.1039/D3OB01312J, Communication
Pradeep Kumar, Deepanshi Saxena, Rahul Maitra, Sidharth Chopra, T. Narender
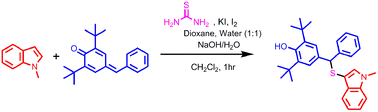
A simple and efficient protocol has been developed to access thioethers by reacting indoles with p-quinone methides using thiourea as a sulfur source.
To cite this article before page numbers are assigned, use the DOI form of citation above.
The content of this RSS Feed (c) The Royal Society of Chemistry
A simple and efficient protocol has been developed to access thioethers by reacting indoles with p-quinone methides using thiourea as a sulfur source.
To cite this article before page numbers are assigned, use the DOI form of citation above.
The content of this RSS Feed (c) The Royal Society of Chemistry