Chem. Sci., 2023, Advance Article
DOI: 10.1039/D3SC04727J, Edge Article
DOI: 10.1039/D3SC04727J, Edge Article
 Open Access
Open Access This article is licensed under a Creative Commons Attribution 3.0 Unported Licence.
This article is licensed under a Creative Commons Attribution 3.0 Unported Licence.Hang T. Dang, Arka Porey, Sachchida Nand, Ramon Trevino, Patrick Manning-Lorino, William B. Hughes, Seth O. Fremin, William T. Thompson, Shree Krishna Dhakal, Hadi D. Arman, Oleg V. Larionov
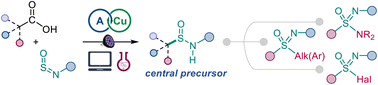
Sulfinamides can now be readily accessed from carboxylic acids and amines in a direct decarboxylative reaction enabled by the kinetically-driven reactivity of sulfinylamines and acridine photocatalysis.
To cite this article before page numbers are assigned, use the DOI form of citation above.
The content of this RSS Feed (c) The Royal Society of Chemistry
Sulfinamides can now be readily accessed from carboxylic acids and amines in a direct decarboxylative reaction enabled by the kinetically-driven reactivity of sulfinylamines and acridine photocatalysis.
To cite this article before page numbers are assigned, use the DOI form of citation above.
The content of this RSS Feed (c) The Royal Society of Chemistry