Faraday Discuss., 2023, 245,467-487
DOI: 10.1039/D3FD00048F, Paper
DOI: 10.1039/D3FD00048F, Paper
Murthy S. Gudipati, Benjamin Fleury, Robert Wagner, Bryana L. Henderson, Kathrin Altwegg, Martin Rubin
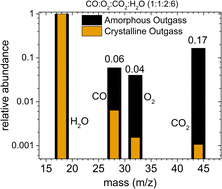
Cometary ice analogs: amorphous H2O ice can trap other molecules up to ∼30% by number, which are outgassed during ice crystallization (130 K to 150 K). Beyond 160 K, crystalline ice sublimes with only a minor fraction (<1%) of other molecules with it.
The content of this RSS Feed (c) The Royal Society of Chemistry
Cometary ice analogs: amorphous H2O ice can trap other molecules up to ∼30% by number, which are outgassed during ice crystallization (130 K to 150 K). Beyond 160 K, crystalline ice sublimes with only a minor fraction (<1%) of other molecules with it.
The content of this RSS Feed (c) The Royal Society of Chemistry