CrystEngComm, 2023, 25,5060-5071
DOI: 10.1039/D3CE00708A, Highlight
DOI: 10.1039/D3CE00708A, Highlight
Steve Scheiner
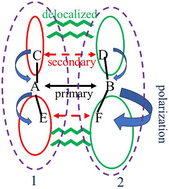
The quantum chemical calculation of the strength of a primary noncovalent bond within a crystal must navigate numerous complicating issues. Choice of geometry, polarizing effects of substituents, and delocalized interactions, must all be considered.
The content of this RSS Feed (c) The Royal Society of Chemistry
The quantum chemical calculation of the strength of a primary noncovalent bond within a crystal must navigate numerous complicating issues. Choice of geometry, polarizing effects of substituents, and delocalized interactions, must all be considered.
The content of this RSS Feed (c) The Royal Society of Chemistry