Environ. Sci.: Atmos., 2023, 3,1541-1551
DOI: 10.1039/D3EA00072A, Paper
DOI: 10.1039/D3EA00072A, Paper
 Open Access
Open Access This article is licensed under a Creative Commons Attribution 3.0 Unported Licence.
This article is licensed under a Creative Commons Attribution 3.0 Unported Licence.Xiaomeng Tian, Ruifeng Zhang, Bo Wei, Yalin Wang, Yongjie Li, Chak K. Chan
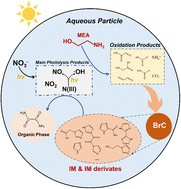
After photolysis, more acidic particles show an increase in pH, while the more neutralized particles show a decrease in pH. NO3− and MEA decay rates are more sensitive to the initial pH than RH. Water-soluble BrC and an organic phase were formed as potential secondary organic aerosols (SOAs).
The content of this RSS Feed (c) The Royal Society of Chemistry
After photolysis, more acidic particles show an increase in pH, while the more neutralized particles show a decrease in pH. NO3− and MEA decay rates are more sensitive to the initial pH than RH. Water-soluble BrC and an organic phase were formed as potential secondary organic aerosols (SOAs).
The content of this RSS Feed (c) The Royal Society of Chemistry