Faraday Discuss., 2023, 245,380-390
DOI: 10.1039/D2FD00180B, Paper
DOI: 10.1039/D2FD00180B, Paper
 Open Access
Open Access This article is licensed under a Creative Commons Attribution 3.0 Unported Licence.
This article is licensed under a Creative Commons Attribution 3.0 Unported Licence.Alexander K. Lemmens, Cameron J. Mackie, Alessandra Candian, Timothy M. J. Lee, Alexander G. G. M. Tielens, Anouk M. Rijs, Wybren Jan Buma
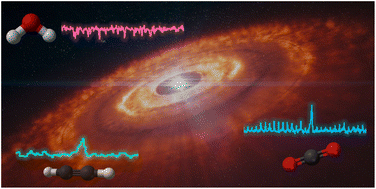
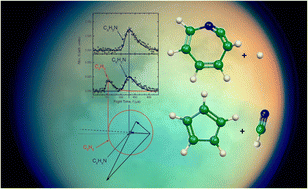
Calculated infrared absorption intensities of PAHs that underlie the interpretation of infrared emission from the ISM are validated with experiments. An adjusted emission model implies that typical PAH sizes are smaller than previously estimated.
The content of this RSS Feed (c) The Royal Society of Chemistry
Calculated infrared absorption intensities of PAHs that underlie the interpretation of infrared emission from the ISM are validated with experiments. An adjusted emission model implies that typical PAH sizes are smaller than previously estimated.
The content of this RSS Feed (c) The Royal Society of Chemistry