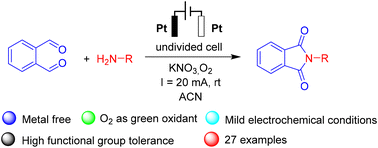
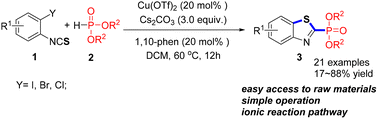
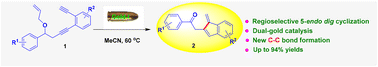
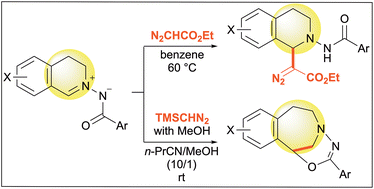
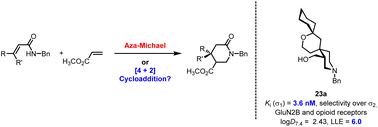
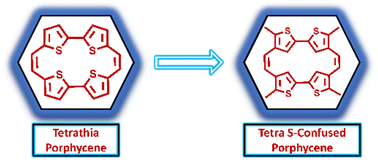
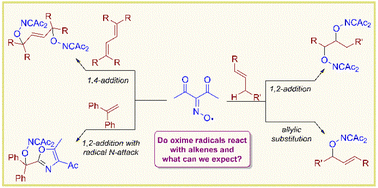
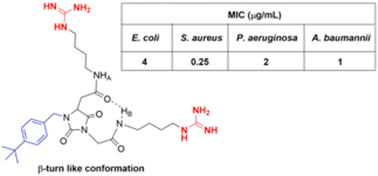
Org. Biomol. Chem., 2023, 21,7717-7723
DOI: 10.1039/D3OB01137B, Paper
DOI: 10.1039/D3OB01137B, Paper
 Open Access
Open Access This article is licensed under a Creative Commons Attribution 3.0 Unported Licence.
This article is licensed under a Creative Commons Attribution 3.0 Unported Licence.Sarah J. Pike, Richard Telford, Louise Male
A versatile and short synthetic route to access a photo-responsive foldamer has been established. The robustness of the reversible conformational switching of the foldamer has been determined using UV/Vis, 1H NMR and circular dichroism spectroscopy.
The content of this RSS Feed (c) The Royal Society of Chemistry
A versatile and short synthetic route to access a photo-responsive foldamer has been established. The robustness of the reversible conformational switching of the foldamer has been determined using UV/Vis, 1H NMR and circular dichroism spectroscopy.
The content of this RSS Feed (c) The Royal Society of Chemistry