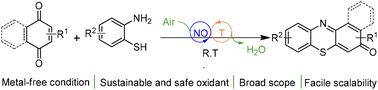
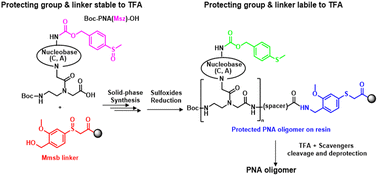
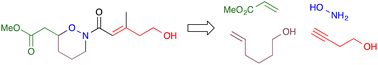
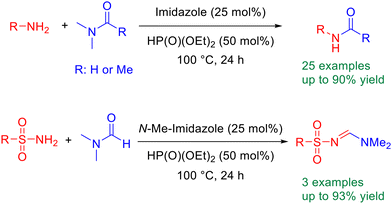
Org. Biomol. Chem., 2023, Advance Article
DOI: 10.1039/D3OB01451G, Paper
DOI: 10.1039/D3OB01451G, Paper
Zhengyu Han, Yu Xue, Xiang Li, Xinzhe Hu, Xiu-Qin Dong, Jianwei Sun, Hai Huang
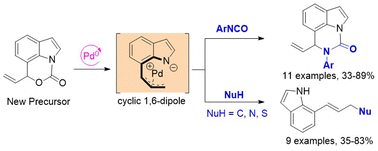
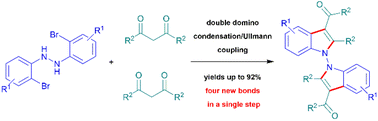
A new kind of indole-fused zwitterionic π-allylpalladium precursor compound has been designed and synthesized.
To cite this article before page numbers are assigned, use the DOI form of citation above.
The content of this RSS Feed (c) The Royal Society of Chemistry
A new kind of indole-fused zwitterionic π-allylpalladium precursor compound has been designed and synthesized.
To cite this article before page numbers are assigned, use the DOI form of citation above.
The content of this RSS Feed (c) The Royal Society of Chemistry

 Open Access
Open Access