Org. Biomol. Chem., 2023, Advance Article
DOI: 10.1039/D3OB01434G, Paper
DOI: 10.1039/D3OB01434G, Paper
Vratta Grover, Mangalampalli Ravikanth
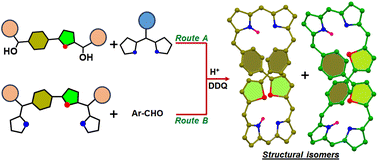
We synthesized rare examples of cis- and trans-isomers of di-p-benzidithiaoctaphyrins by adopting two different synthetic routes, successfully separated the isomers and characterized them using various experimental and theoretical techniques.
To cite this article before page numbers are assigned, use the DOI form of citation above.
The content of this RSS Feed (c) The Royal Society of Chemistry
We synthesized rare examples of cis- and trans-isomers of di-p-benzidithiaoctaphyrins by adopting two different synthetic routes, successfully separated the isomers and characterized them using various experimental and theoretical techniques.
To cite this article before page numbers are assigned, use the DOI form of citation above.
The content of this RSS Feed (c) The Royal Society of Chemistry




 Open Access
Open Access