Org. Biomol. Chem., 2023, Advance Article
DOI: 10.1039/D3OB01309J, Paper
DOI: 10.1039/D3OB01309J, Paper
Aleksandr V. Konovalov, Svetlana G. Churusova, Diana V. Aleksanyan, Ekaterina Yu. Rybalkina, Svetlana A. Aksenova, Alexander S. Peregudov, Zinaida S. Klemenkova, Vladimir A. Kozlov
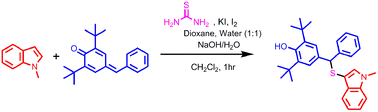
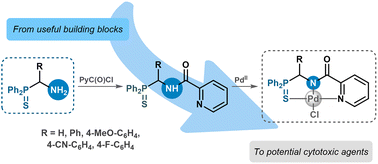
The facile approaches to α-(aminoalkyl)diphenylphosphine sulfides are devised based on simple transformations of readily available precursors. The compounds obtained are used as building blocks for the production of cytotoxic Pd(II) complexes.
To cite this article before page numbers are assigned, use the DOI form of citation above.
The content of this RSS Feed (c) The Royal Society of Chemistry
The facile approaches to α-(aminoalkyl)diphenylphosphine sulfides are devised based on simple transformations of readily available precursors. The compounds obtained are used as building blocks for the production of cytotoxic Pd(II) complexes.
To cite this article before page numbers are assigned, use the DOI form of citation above.
The content of this RSS Feed (c) The Royal Society of Chemistry


 Open Access
Open Access